FDA's Fast-Track for Rexulti Raises Concerns
Por um escritor misterioso
Last updated 09 abril 2025

CMS efforts to reduce use of unnecessary antipsychotics in nursing homes may conflict with marketing efforts for the drug.

FDA rushes approval of dementia drug that quadruples risk of death

FDA approves first drug meant to ease Alzheimer's-linked agitation

FDA's Fast-Track for Rexulti Raises Concerns

Video: Using the REXULTI Savings Card
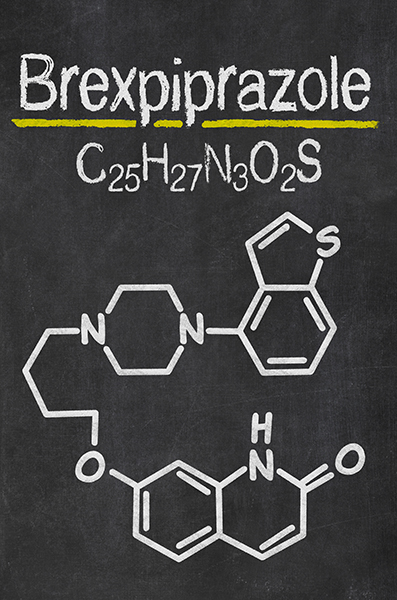
Brexpiprazole Warnings: Side Effects of Rexulti

Fast-Track Drug Approval, Designed for Emergencies, Is Now Routine - WSJ

FDA's Fast-Track for Rexulti Raises Concerns
Video: Using the REXULTI Savings Card

Amgen Triumphs in $27.8 Billion Horizon Buyout Battle
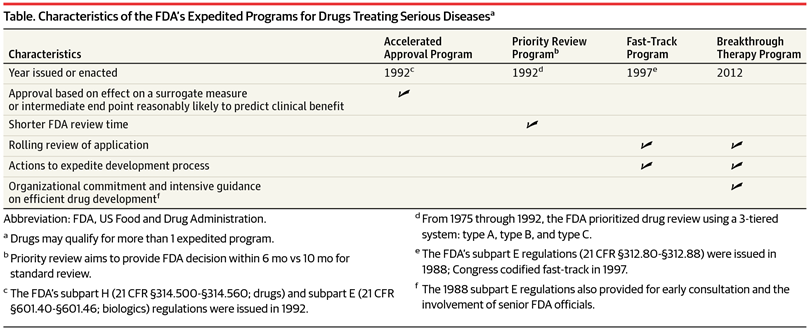
The Science Of A Biotech Valuation: How To Interpret The Value Of FDA Expedited Programs (NASDAQ:IBB)
vtgn20230331_10k.htm

FDA Approves Rexulti For Agitation Associated With Dementia Due To Alzheimer's
Recomendado para você
-
Rexulti Full Prescribing Information, Dosage & Side Effects09 abril 2025
-
Rexulti Full Prescribing Information, Dosage & Side Effects09 abril 2025
-
 REXULTI (brexpiprazole) Tablet09 abril 2025
REXULTI (brexpiprazole) Tablet09 abril 2025 -
rexulti side effects are ruining my life and im on vacation in a forei09 abril 2025
-
 Why REXULTI® (brexpiprazole) agitation that may happen with dementia due to Alzheimer's disease09 abril 2025
Why REXULTI® (brexpiprazole) agitation that may happen with dementia due to Alzheimer's disease09 abril 2025 -
REXULTI® (brexpiprazole), MDD09 abril 2025
-
 Rexulti, the first drug to relieve Alzheimer's emotions - TimesKuwait09 abril 2025
Rexulti, the first drug to relieve Alzheimer's emotions - TimesKuwait09 abril 2025 -
 Rexulti (brexpiprazole) Drug Overview 2019 - Research and Markets09 abril 2025
Rexulti (brexpiprazole) Drug Overview 2019 - Research and Markets09 abril 2025 -
 Rexulti copay card covers generics too : r/pharmacy09 abril 2025
Rexulti copay card covers generics too : r/pharmacy09 abril 2025 -
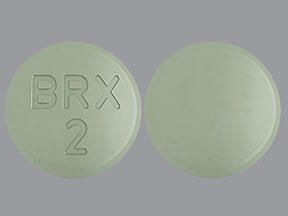 REXULTI 2 MG Oral Tablet09 abril 2025
REXULTI 2 MG Oral Tablet09 abril 2025
você pode gostar
-
 Warzone expert argues FJX Intervention could be a “missed opportunity” in MW3 - Dexerto09 abril 2025
Warzone expert argues FJX Intervention could be a “missed opportunity” in MW3 - Dexerto09 abril 2025 -
/cdn.vox-cdn.com/uploads/chorus_image/image/65141450/1168756251.jpg.0.jpg) Omar Richards Set To Join Pantheon Of Reading FC's England U21 Internationals - The Tilehurst End09 abril 2025
Omar Richards Set To Join Pantheon Of Reading FC's England U21 Internationals - The Tilehurst End09 abril 2025 -
 Carrie-Anne Moss - Wikiwand09 abril 2025
Carrie-Anne Moss - Wikiwand09 abril 2025 -
 The Legend of Zelda: Breath of the Wild Director Has 'Lots of09 abril 2025
The Legend of Zelda: Breath of the Wild Director Has 'Lots of09 abril 2025 -
 9 Best Roblox Games for Kids (Free and Fun!)09 abril 2025
9 Best Roblox Games for Kids (Free and Fun!)09 abril 2025 -
Plastic Memories - Just remembering Isla is still enough for me to cry :'( Admin Furanshis - Kun ▻ Himouto Umaru-chan「Anime this Summer」-due in July 8.09 abril 2025
-
 Technoblade never dies Happy birthday king, Techno, Supportive09 abril 2025
Technoblade never dies Happy birthday king, Techno, Supportive09 abril 2025 -
terminando um desenho da Julia minegirl #juliaminegirl #desenho #fyp #09 abril 2025
-
 Goddess Story - NS-10M01-018 - NTR - [Asuna Yuuki - Sword Art09 abril 2025
Goddess Story - NS-10M01-018 - NTR - [Asuna Yuuki - Sword Art09 abril 2025 -
 I just want to know how they made a working chess game? : r09 abril 2025
I just want to know how they made a working chess game? : r09 abril 2025


