Life Sciences Commissioning, Qualification and Validation
Por um escritor misterioso
Last updated 09 abril 2025


Paperless CQV and Baseline Guide 5
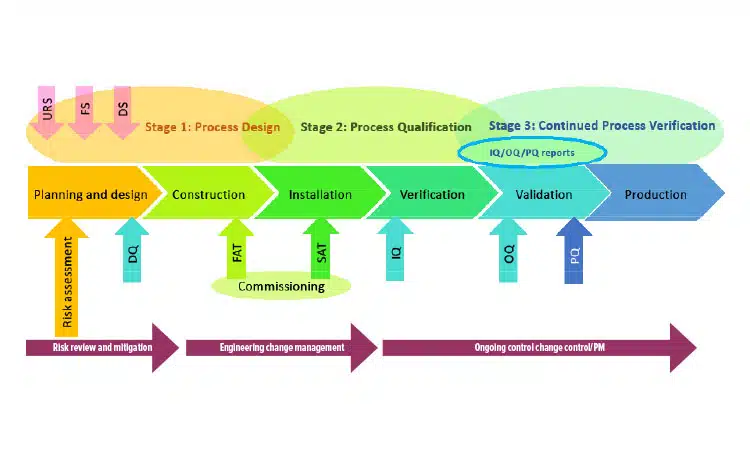
Qualifying a New GMP Facility: From Pitfalls to Best Practices
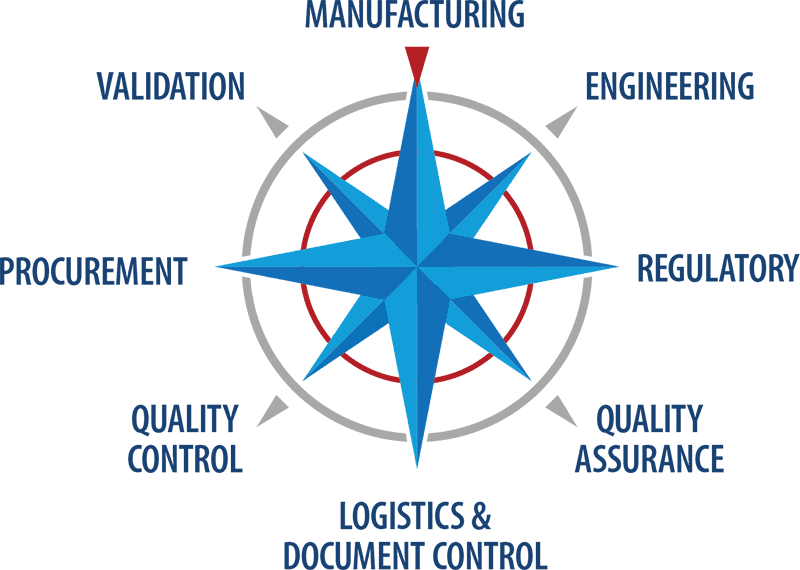
Biopharma Commissioning & Qualification Services, Consultants
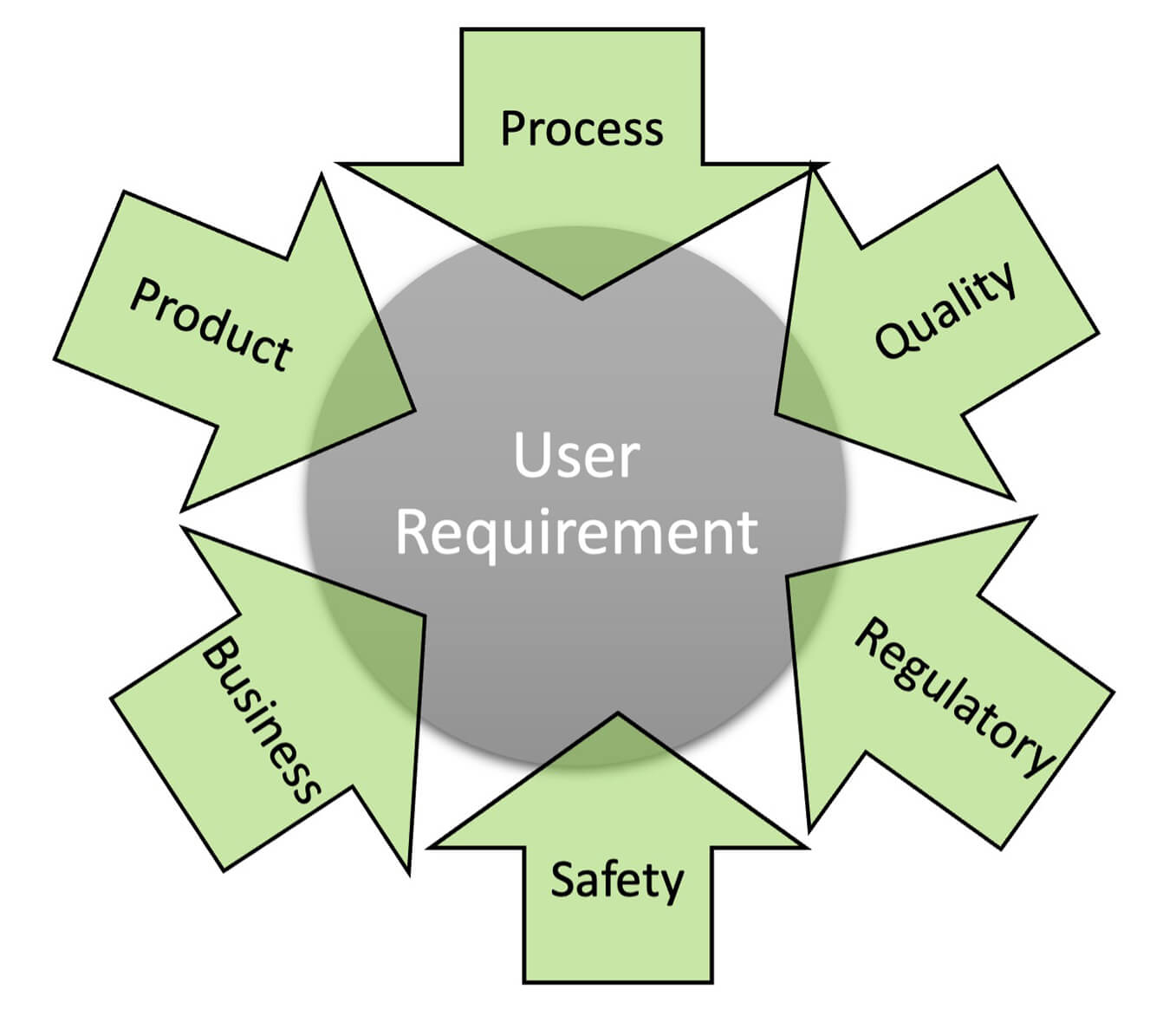
A Successful Q-Model for New mRNA Line Commissioning & Qualification: Pt. 1

Commissioning, Qualification, and Validation

Commissioning Qualification Validation CQV

Commissioning, Qualification & Validation

Commissioning, Qualification & Validation (CQV) Consulting
Apply to Senior Commissioning, Qualification and Validation Engineer-W57 at AEI

Business Case: Commissioning, Qualification, and Validation (CQV) for Facility, Manufacturing, and Laboratory Equipment and Systems - Kvalito

Commissioning and Qualification - VTI Life Sciences
Recomendado para você
-
 Why is this interesting? - The ICQ Edition - by Colin Nagy09 abril 2025
Why is this interesting? - The ICQ Edition - by Colin Nagy09 abril 2025 -
 O ICQ! INSTALAÇÃO, FUNCIONAMENTO E SONS ORIGINAIS PARA VOCÊ!09 abril 2025
O ICQ! INSTALAÇÃO, FUNCIONAMENTO E SONS ORIGINAIS PARA VOCÊ!09 abril 2025 -
![OBSOLETE] Select versions of old ICQ clients work! Here's how to](https://wink.messengergeek.com/uploads/default/original/2X/e/e797ad0ab6709218b412d623390fa2ddc9f2ef7f.png) OBSOLETE] Select versions of old ICQ clients work! Here's how to09 abril 2025
OBSOLETE] Select versions of old ICQ clients work! Here's how to09 abril 2025 -
 Editable Strawberry Baby Shower Invitation Berry Sweet Baby09 abril 2025
Editable Strawberry Baby Shower Invitation Berry Sweet Baby09 abril 2025 -
 Jungle Safari Wild One Birthday Chip Bag Digital File DIY09 abril 2025
Jungle Safari Wild One Birthday Chip Bag Digital File DIY09 abril 2025 -
 Firefighter Invitation Firetruck Invite Firefighter Birthday09 abril 2025
Firefighter Invitation Firetruck Invite Firefighter Birthday09 abril 2025 -
 ICQ Tour09 abril 2025
ICQ Tour09 abril 2025 -
![Cards Cloned/PAYPAL] WWW.Trusted-Best.bz Shop Dumps IST Pin/Card Clone SHOP, PRIVATE SNIFFER, BEST VALIDWe invite sellers,Welcome!, Emv Softwa](https://static.wixstatic.com/media/011f31_f0edb3ba705e4712b539b563e0e4f63e~mv2.png/v1/fill/w_520,h_300,al_c,lg_1,q_85,enc_auto/011f31_f0edb3ba705e4712b539b563e0e4f63e~mv2.png) Cards Cloned/PAYPAL] WWW.Trusted-Best.bz Shop Dumps IST Pin/Card Clone SHOP, PRIVATE SNIFFER, BEST VALIDWe invite sellers,Welcome!, Emv Softwa09 abril 2025
Cards Cloned/PAYPAL] WWW.Trusted-Best.bz Shop Dumps IST Pin/Card Clone SHOP, PRIVATE SNIFFER, BEST VALIDWe invite sellers,Welcome!, Emv Softwa09 abril 2025 -
 ActiveWindows -- ICQ Review09 abril 2025
ActiveWindows -- ICQ Review09 abril 2025 -
 Contact Me09 abril 2025
Contact Me09 abril 2025
você pode gostar
-
![IIT Bombay🏫 [Everything You Need to Know😮🤷♂️]: क्या आप](https://i.ytimg.com/vi/jv1eT0OBVLI/maxresdefault.jpg) IIT Bombay🏫 [Everything You Need to Know😮🤷♂️]: क्या आप09 abril 2025
IIT Bombay🏫 [Everything You Need to Know😮🤷♂️]: क्या आप09 abril 2025 -
 Naruto Uzumaki Hokage Naruto Shippuden: Ultimate Ninja Storm 4 Boruto: Naruto Next Generations PNG - art,…09 abril 2025
Naruto Uzumaki Hokage Naruto Shippuden: Ultimate Ninja Storm 4 Boruto: Naruto Next Generations PNG - art,…09 abril 2025 -
 March, 201209 abril 2025
March, 201209 abril 2025 -
 Shopping Bosque Grão-Pará09 abril 2025
Shopping Bosque Grão-Pará09 abril 2025 -
 New World Straw Hat Legend Codes (October 2023): New Launch09 abril 2025
New World Straw Hat Legend Codes (October 2023): New Launch09 abril 2025 -
 Poop Clicker - Play Online on SilverGames 🕹️09 abril 2025
Poop Clicker - Play Online on SilverGames 🕹️09 abril 2025 -
 Drip Goku Is Now In ASTD! *1 Billion Visits*09 abril 2025
Drip Goku Is Now In ASTD! *1 Billion Visits*09 abril 2025 -
 Spela Brecelj, Basketball Player09 abril 2025
Spela Brecelj, Basketball Player09 abril 2025 -
 Shingeki no Kyojin: Temporada 4 se “divide” en dos partes ¿Qué más sabemos?09 abril 2025
Shingeki no Kyojin: Temporada 4 se “divide” en dos partes ¿Qué más sabemos?09 abril 2025 -
 Hitoribocchi no OO Seikatsu / Tear Jerker - TV Tropes09 abril 2025
Hitoribocchi no OO Seikatsu / Tear Jerker - TV Tropes09 abril 2025