What Does the IRB Review?, Research
Por um escritor misterioso
Last updated 13 abril 2025

Below are the elements the IRB looks for when reviewing research. Federal regulations 45 CFR 46.111 and 21 CFR 56.111 outline the requirements for approval of non-exempt human subjects research. To obtain IRB approval, the IRB must have enough information to determine the criteria in each of the sections below are satisfied.
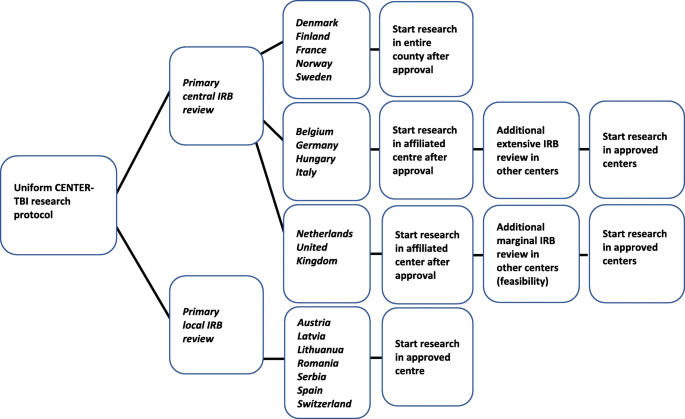
How do 66 European institutional review boards approve one protocol for an international prospective observational study on traumatic brain injury? Experiences from the CENTER-TBI study, BMC Medical Ethics

Activities Requiring IRB Review - Office of Research Support and Compliance
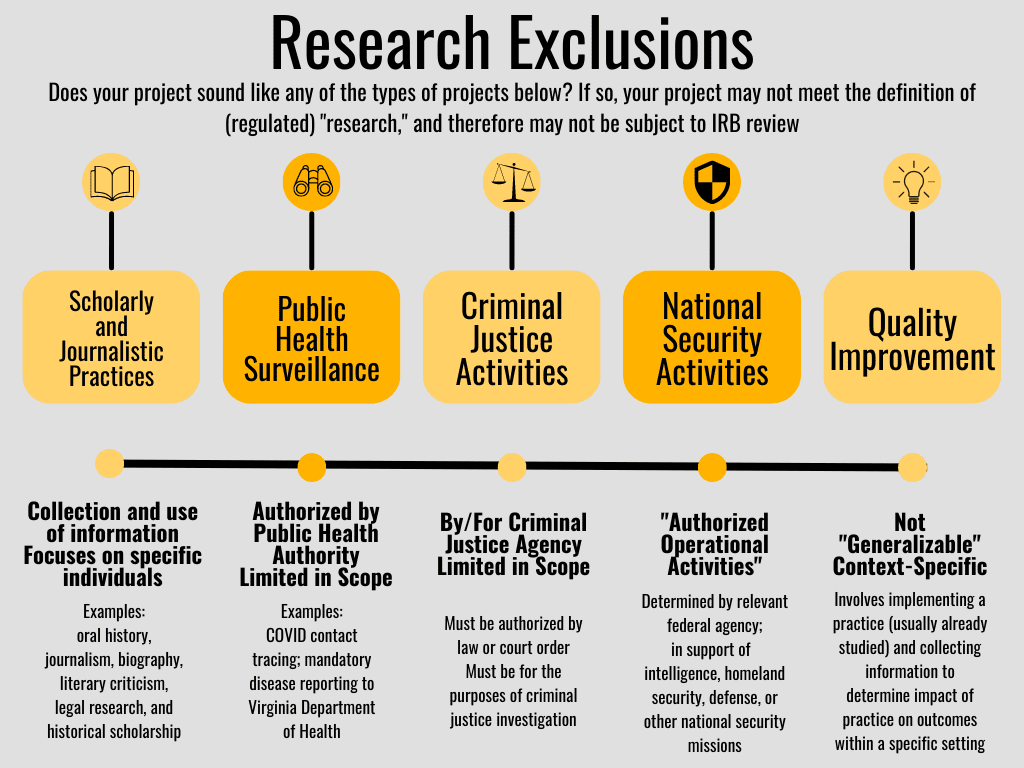
Deep Dives: What is the Difference Between “Exempt” Human Subjects Research, and Projects that are Not Human Subjects Research (NHSR)? – VCU Human Research Protection Program (HRPP) Blog
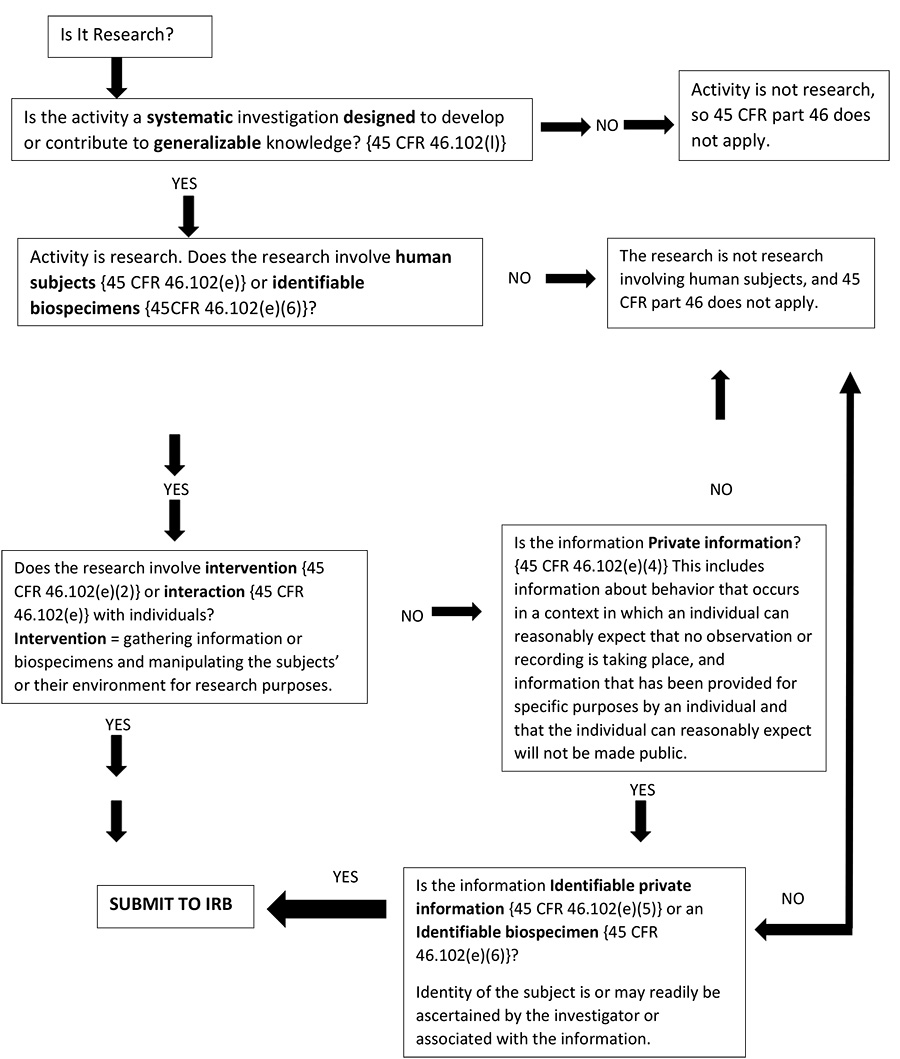
Does Your Research Project Require IRB Review?

What is an IRB?
Institutional Review Board - Barry University, Miami, FL
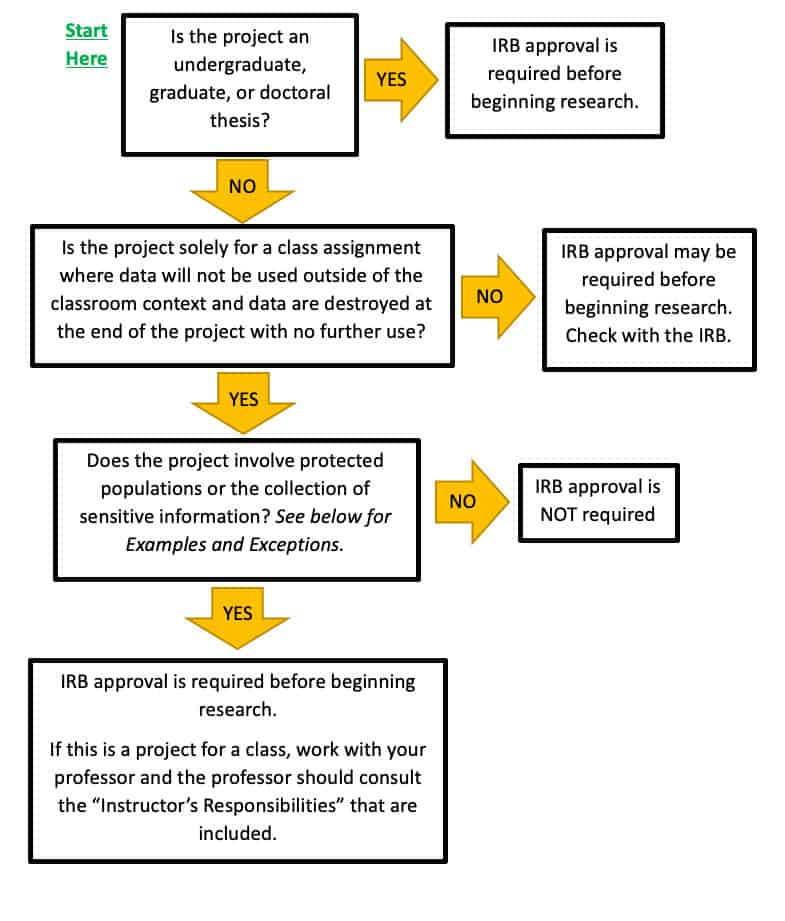
Institutional Review Board - Cairn University

3 Levels of IRB Review Committee on the Use of Human Subjects
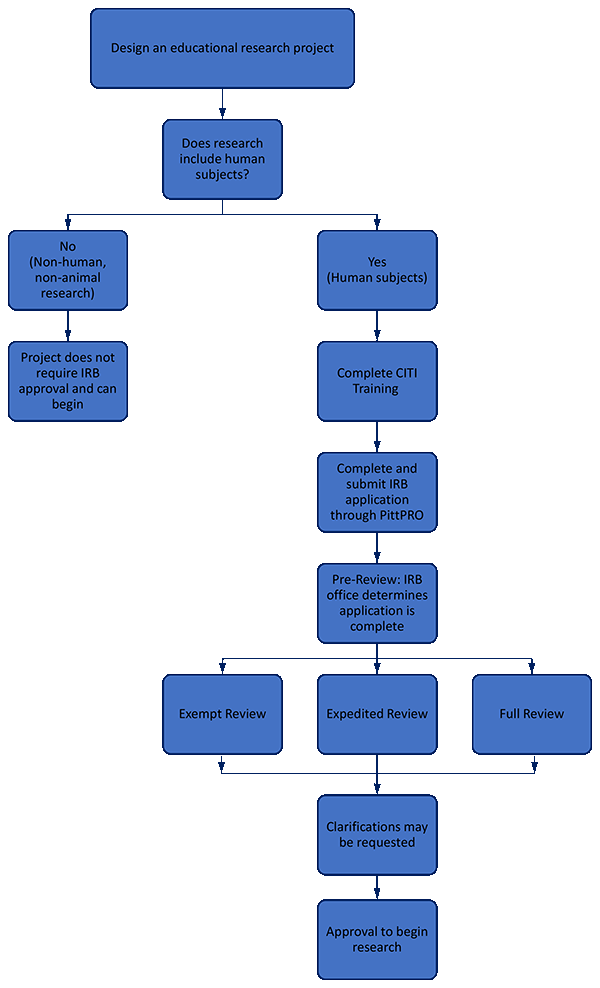
Submitting an IRB application for Educational Research, School of Nursing
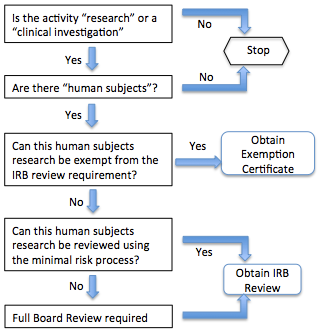
Decision Tree 1

Requirements for Institutional Review Board (IRB) Review and HIPAA Waiver Documentation for RIF DUA Request Submissions
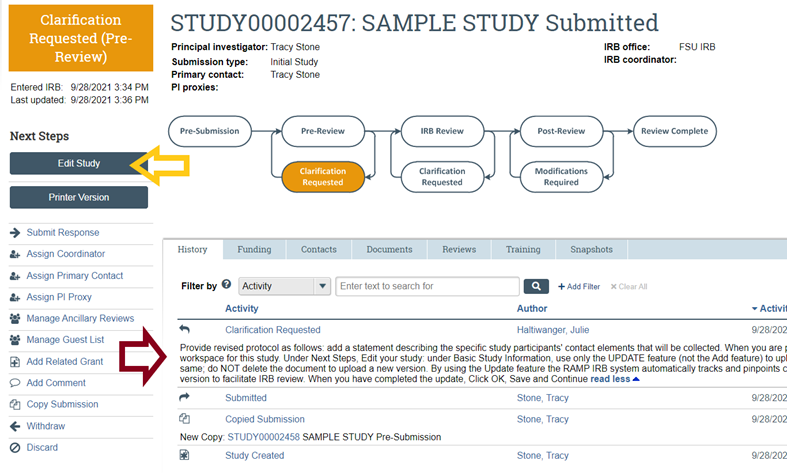
FAQs FSU Office of Research
Recomendado para você
-
Instituto Rio Branco13 abril 2025
-
IRBSL - ♥poussin♥☺Sidi Lakhdar13 abril 2025
-
 QIDIAN Calcomanía de aleación 3D para parachoques delantero de coche, insignia para Cooper S ONE R55 R56 F54 F55 F56 F57 F60 R60 Clubman Hatchback13 abril 2025
QIDIAN Calcomanía de aleación 3D para parachoques delantero de coche, insignia para Cooper S ONE R55 R56 F54 F55 F56 F57 F60 R60 Clubman Hatchback13 abril 2025 -
 DJ TIGER Bodeli Chota Udepur Gujarat Demo Check Roadshow Sound System Setup Sharpy Lasers Truss13 abril 2025
DJ TIGER Bodeli Chota Udepur Gujarat Demo Check Roadshow Sound System Setup Sharpy Lasers Truss13 abril 2025 -
Liz Desmarais, Esq. on LinkedIn: This.Is.Big. Shout out to the entire team at Quantic™ Eulex!13 abril 2025
-
 Institute for Local Self-Reliance – Building Community, Strengthening Economies13 abril 2025
Institute for Local Self-Reliance – Building Community, Strengthening Economies13 abril 2025 -
 Catalog - IRB Advisors, Inc.13 abril 2025
Catalog - IRB Advisors, Inc.13 abril 2025 -
 Overview - Mayo Clinic Research13 abril 2025
Overview - Mayo Clinic Research13 abril 2025 -
 Ishka: SLB returns: Unlevered IRR and NPV analysis13 abril 2025
Ishka: SLB returns: Unlevered IRR and NPV analysis13 abril 2025 -
 fabricio.bobsin@irbsl.com.br13 abril 2025
fabricio.bobsin@irbsl.com.br13 abril 2025
você pode gostar
-
 Ex-São Paulo, Edson Silva é apresentado com pompa na Sérvia - Lance!13 abril 2025
Ex-São Paulo, Edson Silva é apresentado com pompa na Sérvia - Lance!13 abril 2025 -
Here is a quick side by side comparison of Broly Ssj5 next to Goku and Ikari Broly for a size reference of the custom. #broly #brolyssj4…13 abril 2025
-
 Tênis Mad Rats Old School Camel - Store Pesadao13 abril 2025
Tênis Mad Rats Old School Camel - Store Pesadao13 abril 2025 -
 Assistir Kimetsu no Yaiba Filme: Mugen Ressha-hen - Legendado » Anime TV Online13 abril 2025
Assistir Kimetsu no Yaiba Filme: Mugen Ressha-hen - Legendado » Anime TV Online13 abril 2025 -
 Krampus - Madworld Haunted Attractions13 abril 2025
Krampus - Madworld Haunted Attractions13 abril 2025 -
Madworld.co.in13 abril 2025
-
 KNVB ORANJE Zip Hoodie13 abril 2025
KNVB ORANJE Zip Hoodie13 abril 2025 -
 Chaturanga Dandasana Yoga Pose: How to Do It With Perfect Form13 abril 2025
Chaturanga Dandasana Yoga Pose: How to Do It With Perfect Form13 abril 2025 -
Códigos Do Gta 5 de Ps4, PDF13 abril 2025
-
 For 'Fleishman Is in Trouble,' Claire Danes and Jesse Eisenberg Say I Do - The New York Times13 abril 2025
For 'Fleishman Is in Trouble,' Claire Danes and Jesse Eisenberg Say I Do - The New York Times13 abril 2025





