Transcriptional patterns of sexual dimorphism and in host developmental programs in the model parasitic nematode Heligmosomoides bakeri, Parasites & Vectors
Por um escritor misterioso
Last updated 12 abril 2025
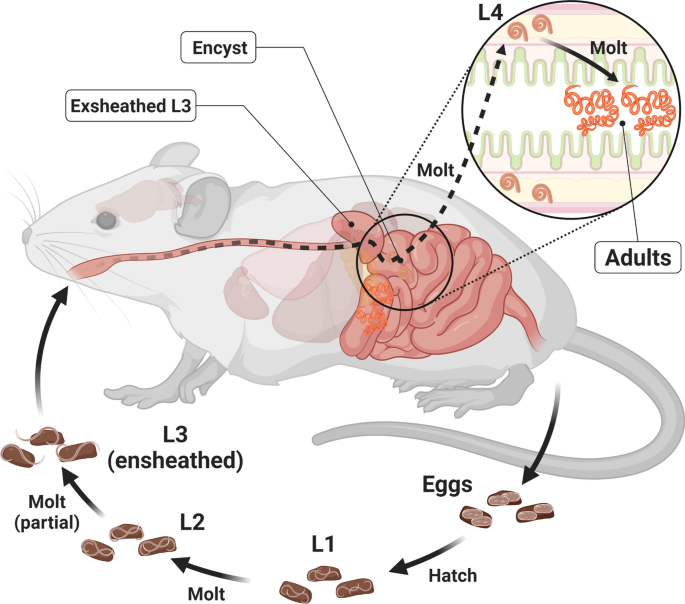
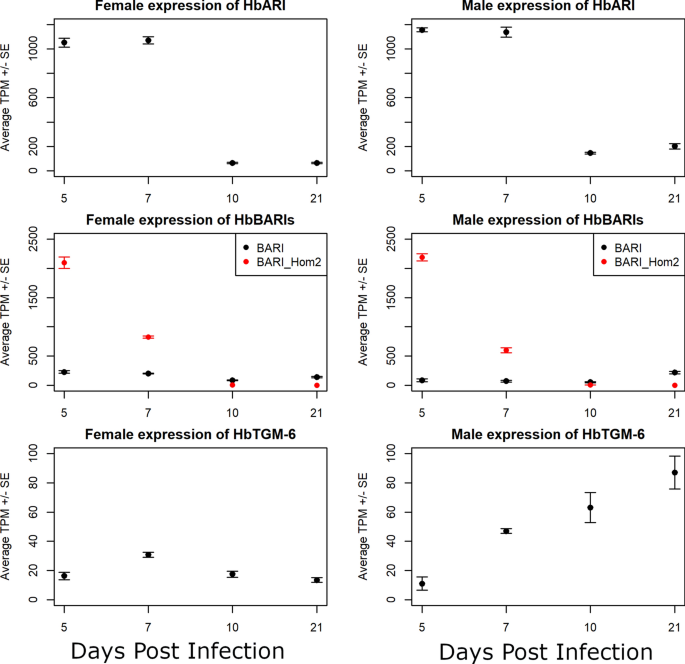
Background Heligmosomoides bakeri (often mistaken for Heligmosomoides polygyrus) is a promising model for parasitic nematodes with the key advantage of being amenable to study and manipulation within a controlled laboratory environment. While draft genome sequences are available for this worm, which allow for comparative genomic analyses between nematodes, there is a notable lack of information on its gene expression. Methods We generated biologically replicated RNA-seq datasets from samples taken throughout the parasitic life of H. bakeri. RNA from tissue-dwelling and lumen-dwelling worms, collected under a dissection microscope, was sequenced on an Illumina platform. Results We find extensive transcriptional sexual dimorphism throughout the fourth larval and adult stages of this parasite and identify alternative splicing, glycosylation, and ubiquitination as particularly important processes for establishing and/or maintaining sex-specific gene expression in this species. We find sex-linked differences in transcription related to aging and oxidative and osmotic stress responses. We observe a starvation-like signature among transcripts whose expression is consistently upregulated in males, which may reflect a higher energy expenditure by male worms. We detect evidence of increased importance for anaerobic respiration among the adult worms, which coincides with the parasite’s migration into the physiologically hypoxic environment of the intestinal lumen. Furthermore, we hypothesize that oxygen concentration may be an important driver of the worms encysting in the intestinal mucosa as larvae, which not only fully exposes the worms to their host’s immune system but also shapes many of the interactions between the host and parasite. We find stage- and sex-specific variation in the expression of immunomodulatory genes and in anthelmintic targets. Conclusions We examine how different the male and female worms are at the molecular level and describe major developmental events that occur in the worm, which extend our understanding of the interactions between this parasite and its host. In addition to generating new hypotheses for follow-up experiments into the worm’s behavior, physiology, and metabolism, our datasets enable future more in-depth comparisons between nematodes to better define the utility of H. bakeri as a model for parasitic nematodes in general. Graphical Abstract

Immune modulation and modulators in Heligmosomoides polygyrus
Transcriptional patterns of sexual dimorphism and in host

The production of excretory-secretory molecules from

Embracing nature's complexity: Immunoparasitology in the wild
Frontiers Host genetic backgrounds: the key to determining
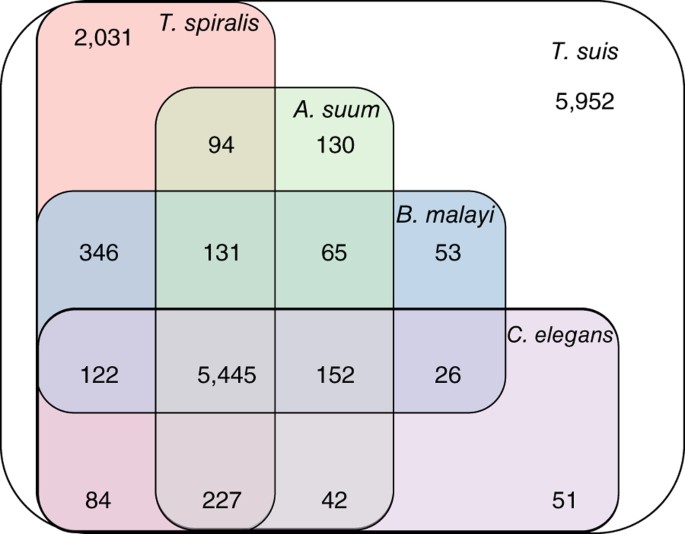
Genome and transcriptome of the porcine whipworm Trichuris suis

The production of excretory-secretory molecules from

The genome and transcriptome of Haemonchus contortus, a key model
PDF) Immunity to the model intestinal helminth parasite
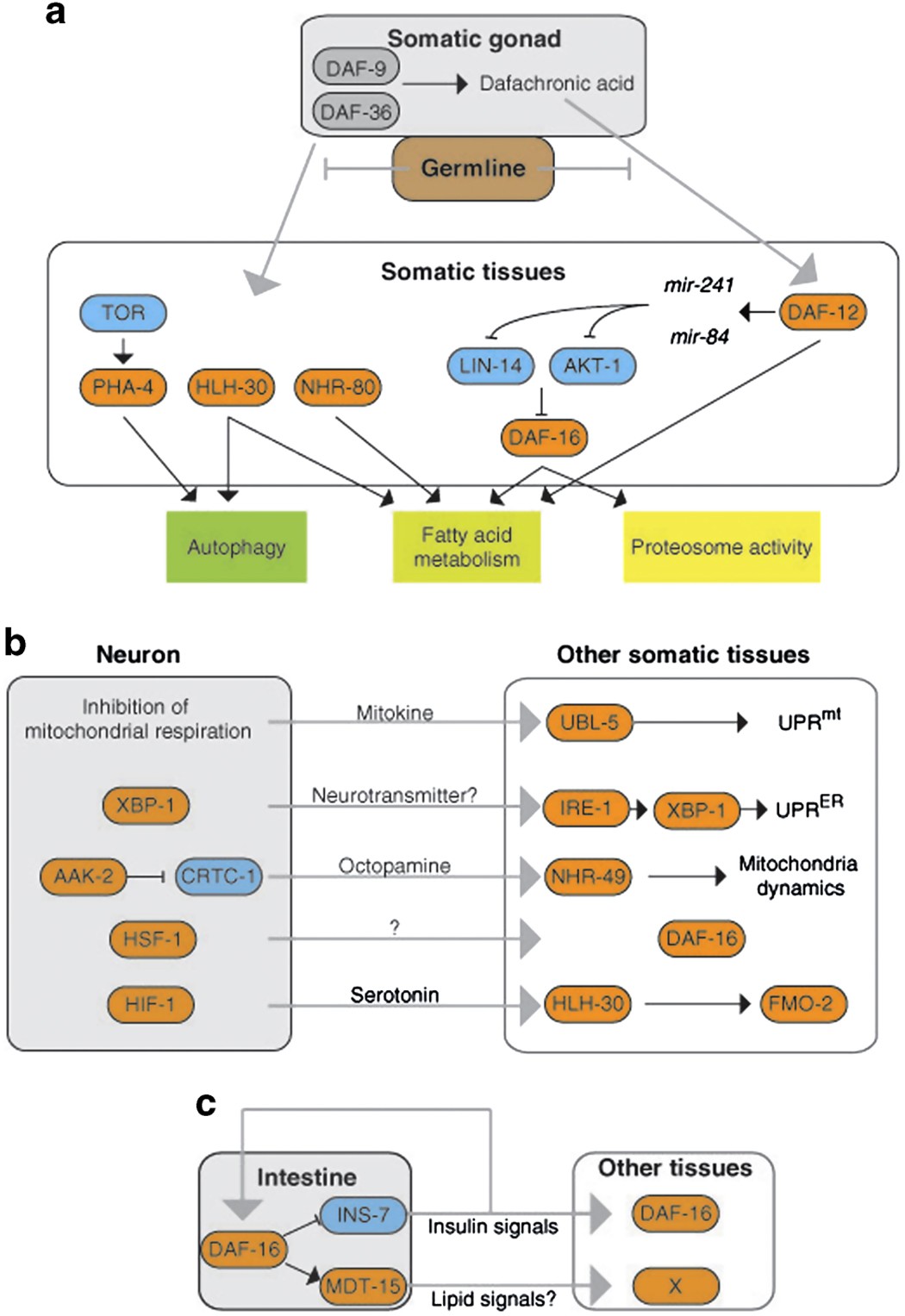
Lifespan-regulating genes in C. elegans
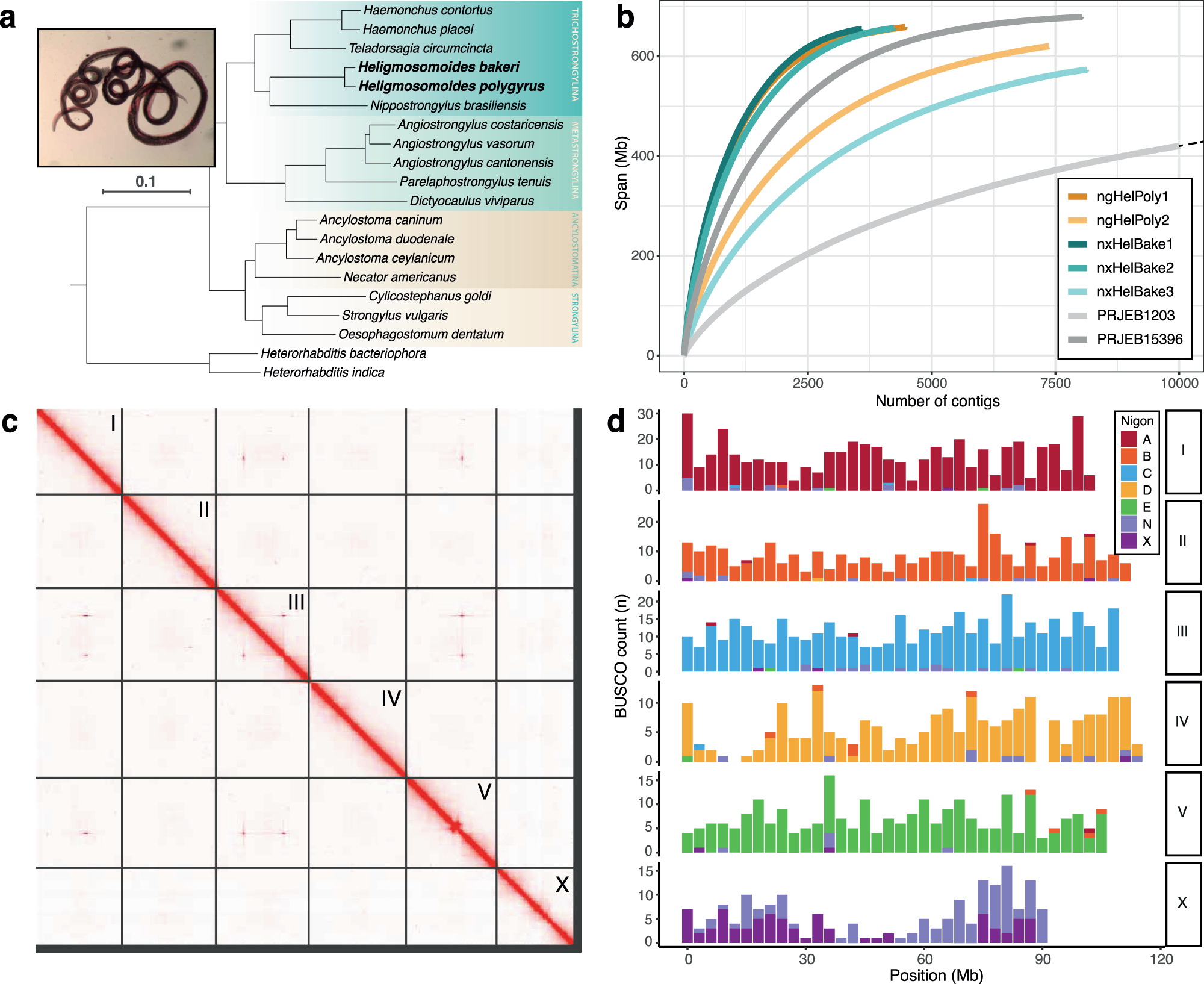
Ancient diversity in host-parasite interaction genes in a model
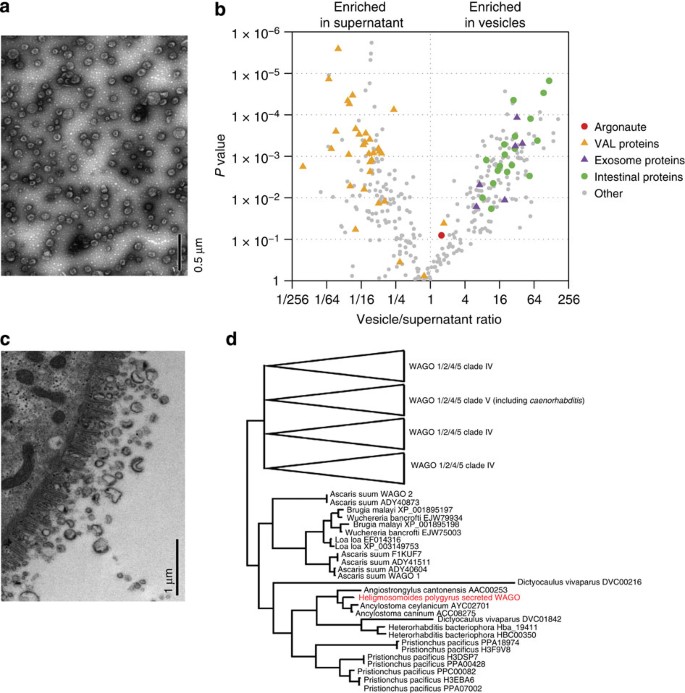
Exosomes secreted by nematode parasites transfer small RNAs to
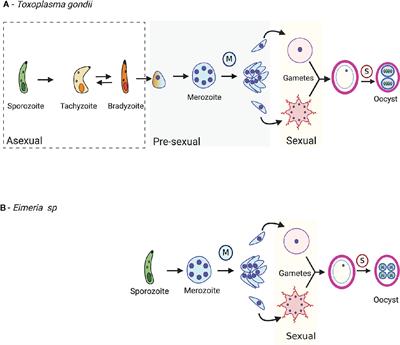
Frontiers Comparisons of the Sexual Cycles for the Coccidian

TGF-β mimic proteins form an extended gene family in the murine
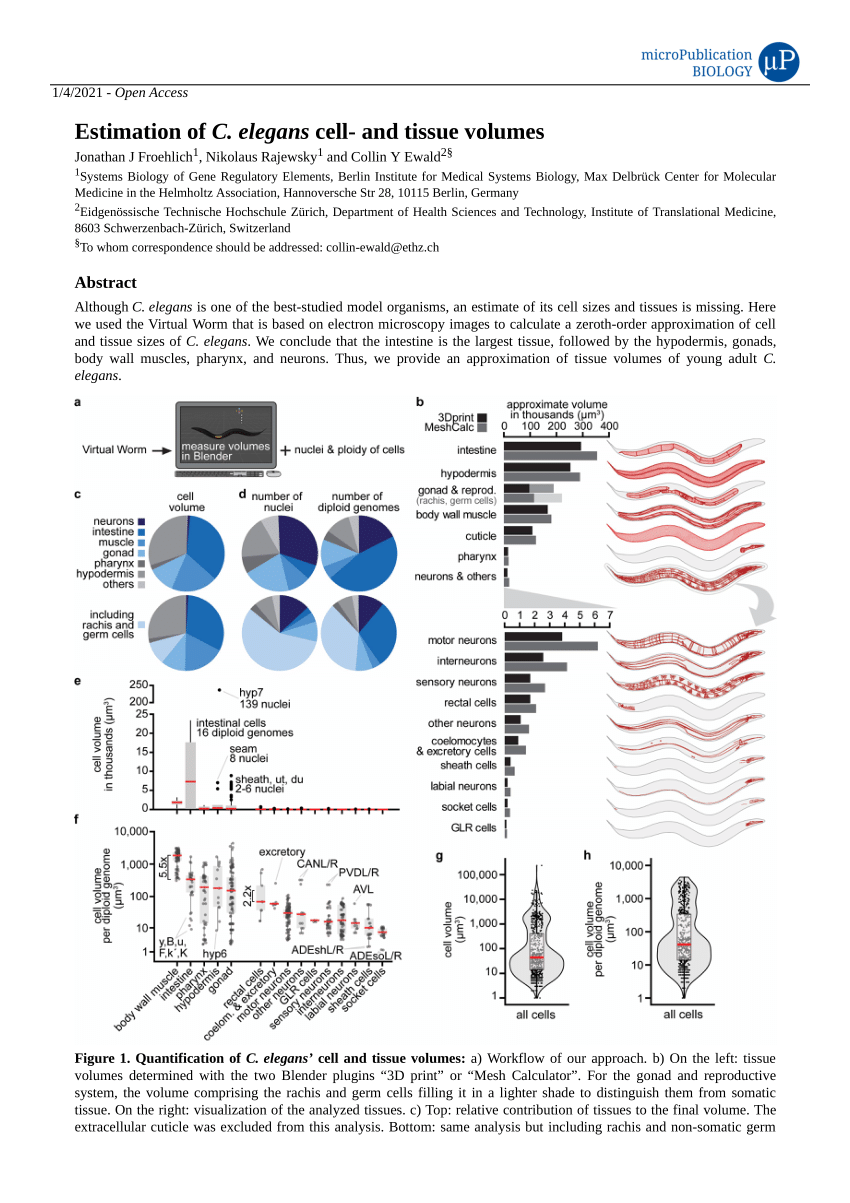
PDF) Estimation of C. elegans cell- and tissue volumes
Recomendado para você
-
 How long does a hamster live? Factors That Influence His Life12 abril 2025
How long does a hamster live? Factors That Influence His Life12 abril 2025 -
 How Long Do Hamsters Live?12 abril 2025
How Long Do Hamsters Live?12 abril 2025 -
 All About Syrian Hamsters - Petopedia12 abril 2025
All About Syrian Hamsters - Petopedia12 abril 2025 -
 Syrian hamster breeding - Wikipedia12 abril 2025
Syrian hamster breeding - Wikipedia12 abril 2025 -
z_syrianhamsterindetail12 abril 2025
-
 New animal model to aid in the development of Covid-19 cure – The Childrens Post of India12 abril 2025
New animal model to aid in the development of Covid-19 cure – The Childrens Post of India12 abril 2025 -
 How I programmed a Virtual Hamster Cage blog - Gamieon - Mod DB12 abril 2025
How I programmed a Virtual Hamster Cage blog - Gamieon - Mod DB12 abril 2025 -
 Gerbil Life Cycle Gerbil, Baby hamster, Cute baby animals12 abril 2025
Gerbil Life Cycle Gerbil, Baby hamster, Cute baby animals12 abril 2025 -
 Hamster Care: The Essential Guide to Ownership, Care, & Training For Your Pet: Pellham, Kate H: 9781511972406: : Books12 abril 2025
Hamster Care: The Essential Guide to Ownership, Care, & Training For Your Pet: Pellham, Kate H: 9781511972406: : Books12 abril 2025 -
 Hamster Care: The Ultimate Guide to Caring for Hamsters12 abril 2025
Hamster Care: The Ultimate Guide to Caring for Hamsters12 abril 2025
você pode gostar
-
 Desenhos para colorir de flor de boca aberta12 abril 2025
Desenhos para colorir de flor de boca aberta12 abril 2025 -
 9-Step Ultimate Off-Page SEO Checklist for Lasting Results - 10Web12 abril 2025
9-Step Ultimate Off-Page SEO Checklist for Lasting Results - 10Web12 abril 2025 -
FC 24, Official Clubs Deep Dive12 abril 2025
-
 Concurso IGP-RS 2017 tem vaga para Engenheiro Civil12 abril 2025
Concurso IGP-RS 2017 tem vaga para Engenheiro Civil12 abril 2025 -
 UNDECEMBER They Need To Nerf This12 abril 2025
UNDECEMBER They Need To Nerf This12 abril 2025 -
SCP-6661 - SCP Foundation12 abril 2025
-
 carta pokémon umbreon vmax - Comprar Cartas Colecionáveis antigas no todocoleccion12 abril 2025
carta pokémon umbreon vmax - Comprar Cartas Colecionáveis antigas no todocoleccion12 abril 2025 -
 Foguinho, Wiki Club Penguin Brasil12 abril 2025
Foguinho, Wiki Club Penguin Brasil12 abril 2025 -
 FINAL FANTASY XI 20TH ANNIVERSARY BEST SELECTION VINYL12 abril 2025
FINAL FANTASY XI 20TH ANNIVERSARY BEST SELECTION VINYL12 abril 2025 -
 YU-NO: A Girl Who Chants Love at the Bound of this World 212 abril 2025
YU-NO: A Girl Who Chants Love at the Bound of this World 212 abril 2025