FDA OKs Cell Therapy to Lower Infection Risk After Stem Cell Transplant
Por um escritor misterioso
Last updated 10 abril 2025

Omidubicel reduced infections in blood cancer patients from 60% to 39% at 100 days posttransplant

Stem Cell Transplantation, Autologous Stem Cell Transplantation
FDA Approves Eflapegrastim-xnst (ROLVEDON) for Chemotherapy
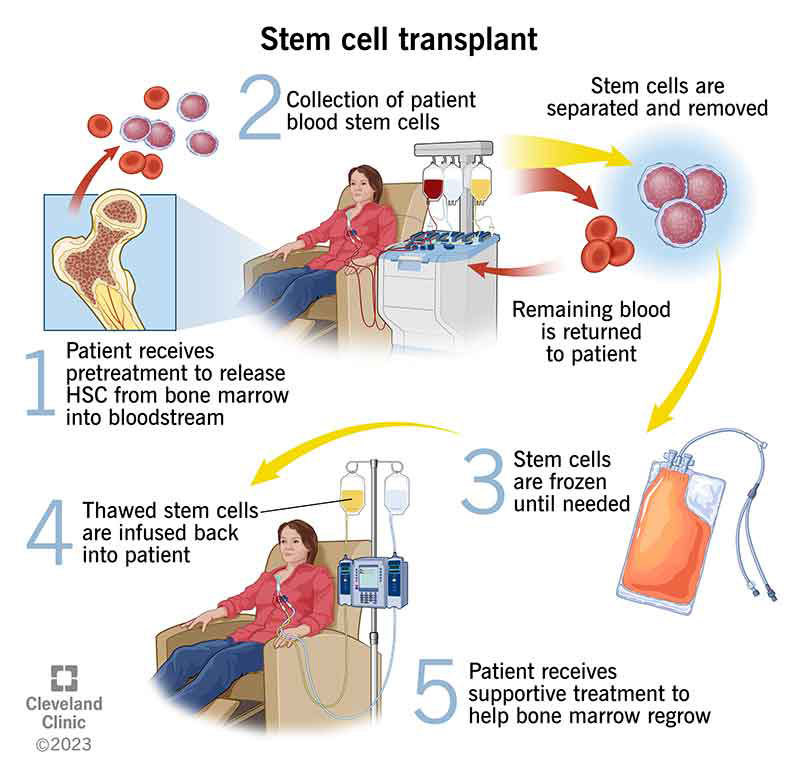
Stem Cell Transplant (Bone Marrow Transplant)
Unusual Clinical Presentation of Clear Cell Sarcoma in a Young Woman
FDA OKs Stem Cell Therapy to Reduce Infection Risk

Weekly reads: FDA nod on new cell therapy, gray hair, pong-playing

The Isolation of the Stem Cell Transplant Ward for NHL

Liver Disease: Induction, Progression, Immunological Mechanisms

Risky Stem-Cell Treatments Come Under F.D.A. Scrutiny — Again
Recomendado para você
-
 Thiago Silva is 39 today - Our Brazilian legend is the finest of wines 💙 : r/chelseafc10 abril 2025
Thiago Silva is 39 today - Our Brazilian legend is the finest of wines 💙 : r/chelseafc10 abril 2025 -
 Duke University Press - Poetics Today10 abril 2025
Duke University Press - Poetics Today10 abril 2025 -
 Egypt receives lists for 13 Israelis and 39 Palestinians for release today10 abril 2025
Egypt receives lists for 13 Israelis and 39 Palestinians for release today10 abril 2025 -
/cdn.vox-cdn.com/uploads/chorus_asset/file/24909597/usa_today_21335839.jpg) 2023 MAC Football Week 8 Game Recap: Toledo Rockets 21, Miami RedHawks 17 - Hustle Belt10 abril 2025
2023 MAC Football Week 8 Game Recap: Toledo Rockets 21, Miami RedHawks 17 - Hustle Belt10 abril 2025 -
/cdn.vox-cdn.com/uploads/chorus_image/image/50510305/img_3185_29064067642_o.0.jpg) Tour Vol. 39, Downtown's New 'Mad Men'-Style Bar, Opening Today - Eater Chicago10 abril 2025
Tour Vol. 39, Downtown's New 'Mad Men'-Style Bar, Opening Today - Eater Chicago10 abril 2025 -
 Palestinian groups reveal names of 39 detainees expected to be released from Israeli jails10 abril 2025
Palestinian groups reveal names of 39 detainees expected to be released from Israeli jails10 abril 2025 -
 Time Frame: RSO Records 39 Years Ago and Today - LAmag - Culture, Food, Fashion, News & Los Angeles10 abril 2025
Time Frame: RSO Records 39 Years Ago and Today - LAmag - Culture, Food, Fashion, News & Los Angeles10 abril 2025 -
 39 Problems, bar and restaurant in Albion, reopens on Main Street10 abril 2025
39 Problems, bar and restaurant in Albion, reopens on Main Street10 abril 2025 -
 39/366 what is in my dance bag, Today started my Belly Danc…10 abril 2025
39/366 what is in my dance bag, Today started my Belly Danc…10 abril 2025 -
 israel Hamas War, Gaza Hospital Babies: Heartbreaking Image From Gaza Shows Babies Laid Side By Side For Warmth10 abril 2025
israel Hamas War, Gaza Hospital Babies: Heartbreaking Image From Gaza Shows Babies Laid Side By Side For Warmth10 abril 2025
você pode gostar
-
vegeta descobre q trunks é seu filho|Pesquisa do TikTok10 abril 2025
-
Agachamento Sumô Abrindo Passada Cross on Vimeo10 abril 2025
-
 The Legend of Zelda: Majora's Mask 3D - IGN10 abril 2025
The Legend of Zelda: Majora's Mask 3D - IGN10 abril 2025 -
 Kitchen wall art, Retro kitchen decor, Kitchen quotes, Food prints, Funny kitchen art, gift for cook, chop it like it's hot print10 abril 2025
Kitchen wall art, Retro kitchen decor, Kitchen quotes, Food prints, Funny kitchen art, gift for cook, chop it like it's hot print10 abril 2025 -
 BEST Towers in Each CATEGORY - Tower Defense Simulator - BiliBili10 abril 2025
BEST Towers in Each CATEGORY - Tower Defense Simulator - BiliBili10 abril 2025 -
Dragon Ball Super A Terra explode?! O Kame Hame Ha Final! - Assista na Crunchyroll10 abril 2025
-
 Wolfenstein: The New Order Game Guide10 abril 2025
Wolfenstein: The New Order Game Guide10 abril 2025 -
 The Outer Worlds 2 Needs To Set Itself Apart In One Big Way10 abril 2025
The Outer Worlds 2 Needs To Set Itself Apart In One Big Way10 abril 2025 -
 Kanpeki Ojou-sama No Watakushi Ga Dogeza De Mazo Ochisuru Choroin Na Wakenai Desu Wa! (TV Series 2019-2020) - Imagens de fundo — The Movie Database (TMDB)10 abril 2025
Kanpeki Ojou-sama No Watakushi Ga Dogeza De Mazo Ochisuru Choroin Na Wakenai Desu Wa! (TV Series 2019-2020) - Imagens de fundo — The Movie Database (TMDB)10 abril 2025 -
 Os Cavaleiros do Zodíaco Filme 2: A Grande Batalha dos Deuses10 abril 2025
Os Cavaleiros do Zodíaco Filme 2: A Grande Batalha dos Deuses10 abril 2025
